Emission spectra are produced by excited atoms in a gas. Gas like hydrogen can be excited by heating or passing an electric discharge through it.
- Emission spectrums consist of discrete lines on a dark background.

- The emission spectrum can be observed by passing the EM radiated from the excited gas through a prism or diffraction grating to separate the discrete wavelengths.

- Andres Angstrom had measured the wavelengths of the four visible spectral lines in the Hydrogen emission spectrum.
- Balmer found an equation that enabled him to calculate these wavelengths
- Balmer’s equation for the wavelength was as follows :
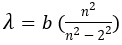
- where n , could be changed to get the various lines
- b was empirically found to be 364.56 nm
- Rydberg modified Balmer’s equation to the following form :
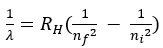
- where Balmer’s equation would be the special case with
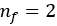
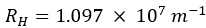 is known as the Rydberg constant.
is known as the Rydberg constant.
- where Balmer’s equation would be the special case with
Extract from Physics Stage 6 Syllabus © 2017 NSW Education Standards Authority (NESA)
