the Geiger–Marsden experiment
The Geiger-Marsden Experiments were conducted by Hans Geiger and Ernest Marsden, between 1908 and 1913.
- The experiments were conducted under the guidance of Ernest Rutherford, and sometimes called Rutherford’s Gold Foil Experiment.
- Thomson had proposed a model of an atom , where atoms were positively charged fluid with negatively charged electrons scattered throughout the atom
- Rutherford suggested that Marsden and Geiger conduct an experiment by firing α-particles(identical to He-4 nucleus) at a very thin gold foil and analyse the results, to study the structure of atom.
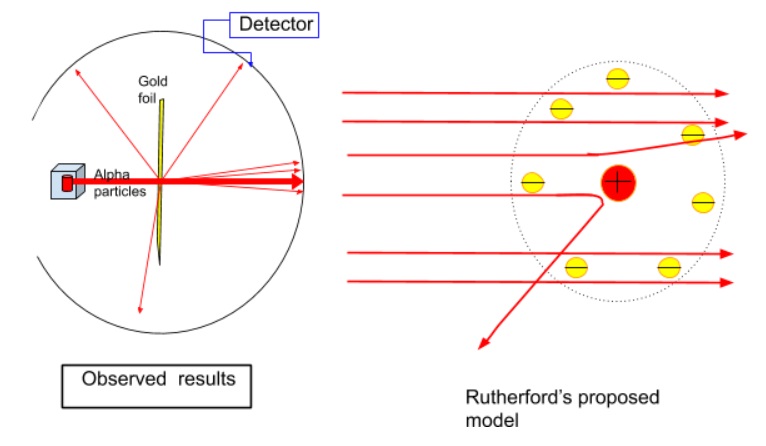
- The expected results, if Thomson’s plum-and-pudding model of an atom were true, would be that most of the alpha particles pass the gold foil with very small deflections.

- The observed results were quite different.
- Marsden observed that 99% of the particles passed through the model very little deflection, if at all.
- There were a tiny fraction of alpha particles that got deflected at angles more 90 degrees.
- There were some observations of alpha particles which were deflected at 180 degrees, right back at the source!
Rutherford’s atomic model
Based on the observations of the experiment Rutherford’s proposed a new model of atom.
- Rutherford realised that the results of the experiment were similar to a charge body interaction.
- He concluded that :
- since most alpha particles passed without any deflection, most of the atom must be empty space.
- since there were observations where alpha particles rebounded with almost 180 degree deflections and few more than 90 degree deflections, there must be a small portion of the atom with a very high charge, that produced such strong deflections.
- Rutherford proposed a model of nuclear atom .
- In this model, electrons orbited around the outside of a much more massive positive centre or ‘nucleus’ much as the planets orbit the sun.
- This large nucleus contained 99.9% of the atom’s overall mass.
Although Rutherford’s model was able to explain most of the experimental observations, it had a few shortcomings:
- If the electrons were orbiting the nucleus in the center, they would have to accelerate and thus give out electromagnetic radiation and energy according to Maxwell’s Theory of electromagnetism.
- As electrons lose energy and spiral inwards, the frequency of EM waves emitted would steadily change. This was never observed.
- Electrons according to this model would lose energy in the form of Electromagnetic radiation and thus the atom would collapse.
Chadwick’s discovery of the neutron (ACSPH026)
After proposing the the model of nuclear atom , Rutherford realised the need for a particle in nucleus without any positive or negative charge.
- He called this particle a neutron.
- Rutherford and James Chadwick carried out experiments in the Cavendish laboratory to experimentally verify the existence of neutron in the 1920s without much success.
- At this time it was assumed that the neutral particle was just a combination of proton and electron in the nucleus.
- Slowly experimental evidence and Heisenberg’s uncertainty principle made the existence of electron very unlikely within the nucleus.
- In 1930 Walter Bothe and Herbert Backer found that if the energetic alpha particles emitted from polonium fell on Beryllium , it produced highly penetrative radiations.
- This unknown radiation was uncharged and much more penetrating than gamma rays, thus the radiation was unlikely to be gamma rays and results were difficult to interpret.
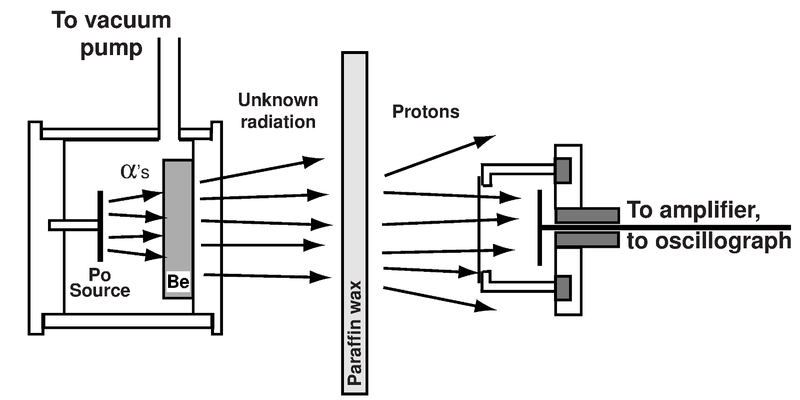
- Two years later Irène Joliot-Curie and Frédéric Joliot in Paris showed that if this unknown radiation fell on paraffin wax, it ejected protons of very high energy (5 MeV).
- From energy and momentum considerations, a gamma ray would have to have impossibly high energy (50 MeV) to scatter a massive proton.
- On hearing of the Paris results, Rutherford and Chadwick at Cavendish Laboratory realised it wasn’t gamma rays, but some other electrically neutral radiation.
- Chadwick recreated the experiment himself. He studied the emitting protons from paraffin and the impact of the unknown radiation on various other gases.
- From his observations , Chadwick concluded that the unknown radiation was composed of uncharged particles of mass about the same as protons.
- Chadwick had thus discovered the proposed particle neutron, for which he received the Nobel prize in 1935.
Extract from Physics Stage 6 Syllabus © 2017 NSW Education Standards Authority (NESA)
